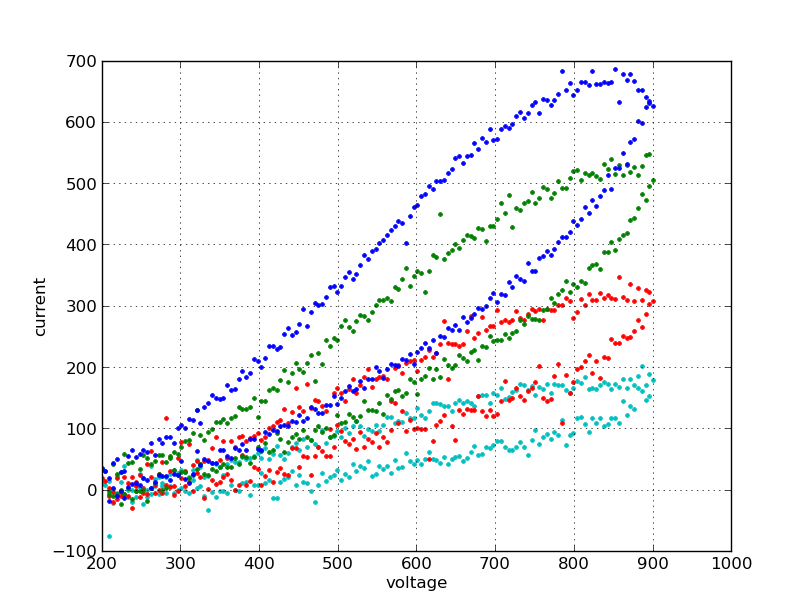
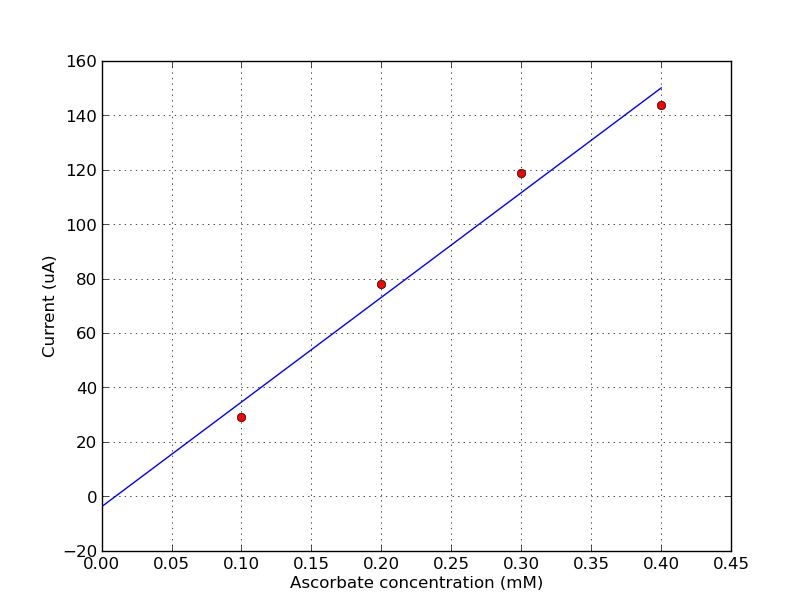
Ascorbate standard curve¶
Reagents & Equipment¶
Cyclic voltammetry parameters¶
- Start: 200 mV
- Stop: 900 mV
- Slope: 50 mV/sec
- Sample rate: 5 mV/sample
- Cycles: 1
Method¶
- Prepare a fresh stock of 100 mM ascorbate in 0.1M KCl. [3]
- Dilute in 0.1M KCl to make a 1 mM ascorbate solution. [4]
- Using 1 mL microcentrifuge tubes, prepare the following standard solutions of ascorbate
| Ascorbate concentration (mM) | Volume of 1mM stock (mL) | Volume of 0.1M KCl (mL) |
|---|---|---|
| 0.1 | 0.10 | 0.9 |
| 0.2 | 0.20 | 0.8 |
| 0.3 | 0.30 | 0.7 |
| 0.4 | 0.40 | 0.6 |
- Connect the screen printed adapter ver A to the CheapStat and insert a screen printed electrode
- Pipette 50 uL of 0.1M KCl onto the screen printed electrode
- Take a background reading, save data
- Next, pipette 50 uL of the first 0.1 mM ascorbate standard onto the screen printed electrode and run a measurement.
- Repeat for all ascorbate concentrations, taking a new background reading between each ascorbate measurement.
Data Analysis¶
To create a standard curve, subtract the background values from the data. Determine the current at 600mV for all samples. Plot a graph of ascorbate concentration (x-axis) versus measured current.
Footnotes
| [1] | To prepare 500 mL of a 0.1M KCl solution: transfer 33 mL of 3M KCl stock (LabChem Part # LC18795-1) into a 500 mL volumetric flask. Fill to line with distilled or deionized water. Place stopper in flask and invert several times to mix. |
| [2] | LabChem (www.labchem.com). Part # LC11530-9. $29.30 for 100 g. |
| [3] | Example: 0.176g ascorbate in 10mL of 0.1M KCl = 100 mM stock |
| [4] | Example, 100uL of 100mM ascorbate stock in 10 mL of 0.1M KCl = 1mM solution |